that exerts its effects by selectively blocking a sodium channel critical for pain signaling in the peripheral nervous system. In the following pages, we shall explore the biological underpinnings of pain transmission, the detailed structure and function of voltage‐gated sodium channels, the precise molecular interactions by which Journavx intervenes in this process, and finally, a comparative discussion of this mechanism versus traditional opioid-based therapies.
Introduction and Overview of Pain Transmission
Dear Reader,
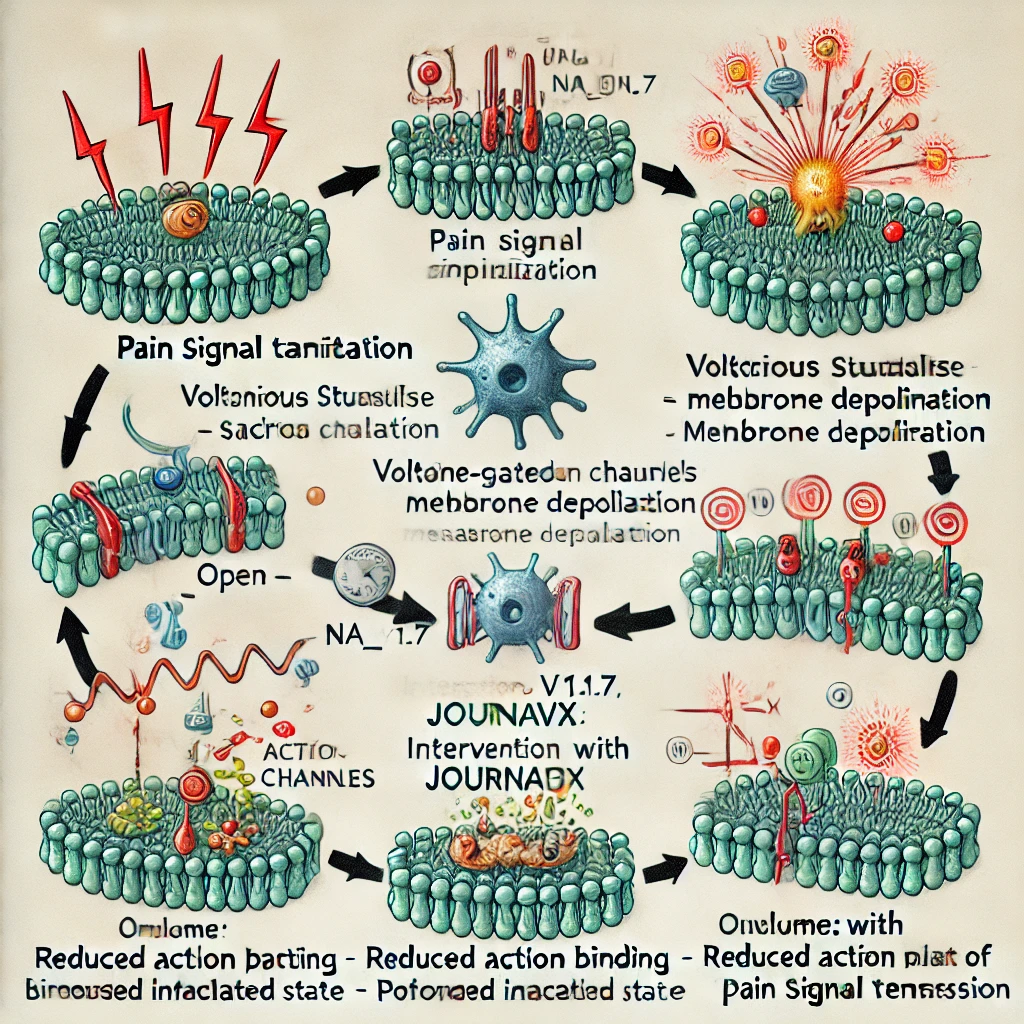
Pain is a multifaceted physiological phenomenon that serves as an essential warning system for potential or actual tissue damage. The conventional understanding of pain transmission involves the transduction, transmission, and perception of noxious stimuli through complex neural pathways. Central to this process is the role played by voltage-gated sodium channels (VGSCs) located on the membranes of peripheral sensory neurons. These channels are instrumental in the generation and propagation of action potentials—the electrical impulses that convey information from the periphery to the central nervous system.
Historically, the management of acute pain in the clinical setting has often relied on opioid analgesics, which primarily function by binding to opioid receptors within the brain and spinal cord. However, the well-documented risk of dependence and abuse associated with opioids has precipitated a search for alternative pharmacological agents. Journavx represents a promising innovation in this context. Unlike opioids, Journavx operates by directly inhibiting the activity of a specific sodium channel subtype implicated in pain signaling. This selective blockade prevents the initiation and propagation of action potentials in nociceptive fibers, thereby mitigating the transmission of pain signals to the brain.
To summarize, this page has introduced the physiological significance of pain and the pivotal role of sodium channels in neural excitability. In the pages that follow, we will delve deeper into the structure-function relationship of these channels and elucidate how Journavx’s mechanism of action offers a potentially safer and equally efficacious alternative to opioid-based analgesia.
The Biology of Voltage-Gated Sodium Channels and Pain Signalling
The physiology of pain transmission hinges on the rapid and precise functioning of voltage-gated sodium channels. These transmembrane proteins are essential for converting electrical signals into the nerve impulses that define neural communication. In nociceptive neurons, specific subtypes—often denoted as Na_V1.7, Na_V1.8, and Na_V1.9—play critical roles in the propagation of pain signals. Let us examine the structure and function of these channels in more detail.
- Channel Architecture and Functional Domains:
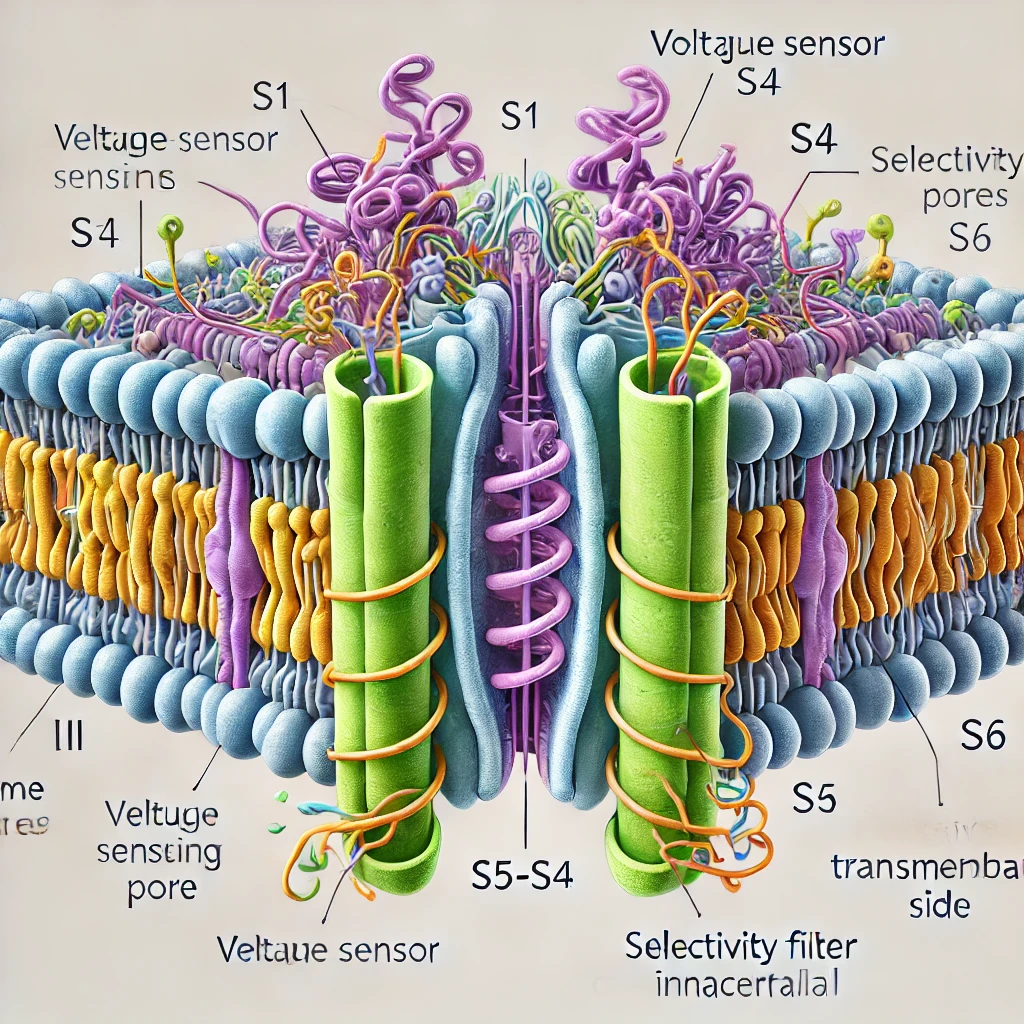
A typical voltage-gated sodium channel is comprised of a large α-subunit that forms the pore through which sodium ions traverse, along with auxiliary β-subunits that modulate channel kinetics and membrane expression. The α-subunit is organized into four homologous domains (I–IV), each containing six transmembrane segments (S1–S6). The S4 segments, rich in positively charged residues, act as voltage sensors that trigger conformational changes during depolarization. Notably, the loop between segments S5 and S6 within each domain contributes to the formation of the ion-selective pore. - Generation and Propagation of Action Potentials:
In resting neurons, sodium channels remain in a closed state. However, when a noxious stimulus depolarizes the membrane beyond a threshold, these channels open, allowing an influx of sodium ions. This rapid ionic movement further depolarizes the neuron, initiating an action potential. The wave of depolarization propagates along the axon, ultimately reaching the dorsal horn of the spinal cord and being relayed to higher brain centers for pain perception. - Specificity in Pain Neurons:
Certain sodium channel subtypes, such as Na_V1.7, have been shown to be especially critical in pain pathways. Genetic studies and clinical observations have underscored the importance of these channels—mutations leading to either loss or gain of function can result in conditions of insensitivity to pain or, conversely, chronic pain syndromes. To assist in visualizing these concepts, please refer to the following simplified schematic representation of a voltage-gated sodium channel:
Extracellular
│
[S1] [S2] [S3]
┌────────────┬────────────┐
│ Voltage Sensor │
└────────────┴────────────┘
[S4] (Sensor Movement)
│
┌────[Pore Domain]────┐
│ (S5-S6 loops) │
└─────────────────────┘
│
IntracellularThis diagram provides a conceptual overview of the channel’s domains, highlighting the critical regions involved in voltage sensing and ion conduction.
Journavx and Its Selective Sodium Channel Blockade
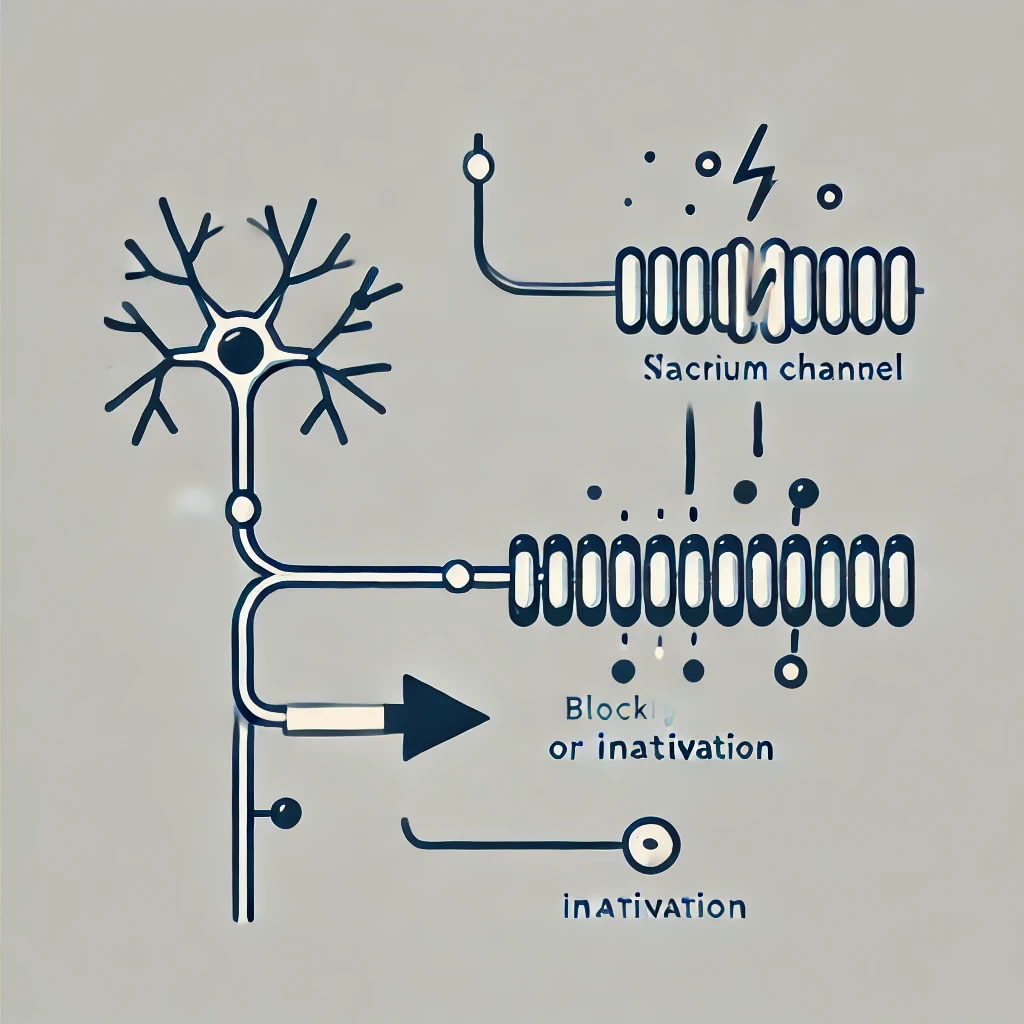
Journavx distinguishes itself as a non-opioid analgesic through its precise pharmacological targeting of a sodium channel subtype integral to pain transmission. The drug’s mechanism can be summarized in the following detailed steps:
- *Selective Binding to the Sodium Channel: *
Journavx is designed to interact with a specific binding pocket on the sodium channel that is preferentially expressed in nociceptive neurons. Research suggests that its binding affinity is markedly higher for channels such as Na_V1.7, which have been implicated in the amplification of pain signals. By selectively associating with this channel, Journavx avoids the systemic effects typically associated with non-selective sodium channel blockers. - *Stabilization of the Inactivated State: *
Upon binding, Journavx induces a conformational stabilization of the channel in its inactivated state. During the normal cycle of activation and inactivation, sodium channels briefly enter a refractory period during which they cannot be reactivated immediately. Journavx prolongs this state, thereby reducing the frequency of channel reopening and, consequently, the number of action potentials generated in response to painful stimuli. - *Inhibition of Action Potential Propagation: *
The selective blockade results in the attenuation of depolarizing currents. With fewer sodium ions entering the neuron, the generation and propagation of action potentials are significantly dampened. This effect is particularly beneficial in the context of acute pain management, where a rapid yet controlled reduction in pain signal transmission is desired. - *Diagrammatic Representation of Journavx Action: * The following ASCII diagram is intended to provide a conceptual visualization of Journavx’s interaction with a voltage-gated sodium channel:
[Nociceptive Neuron Membrane]
┌─────────────────────────┐
│ Voltage-Gated │
│ Sodium Channel │
│ │
│ ┌─────────────┐ │
│ │ Journavx │◄─────Binding Site
│ └─────────────┘ │
└─────────────────────────┘
↑ ↓
Normal Activation Prolonged InactivationIn this schematic, Journavx is depicted as occupying a critical binding site that enforces a sustained inactivated conformation of the sodium channel. The result is a reduction in the channel’s ability to support rapid action potential firing.
- *Molecular Interactions and Structural Considerations: * On a molecular level, Journavx is believed to engage in hydrophobic interactions and hydrogen bonding with amino acid residues lining the channel’s pore region. These interactions are highly specific, ensuring that the blockade does not interfere with sodium channels in other tissues—thereby minimizing off-target effects and enhancing the drug’s safety profile. The precision with which Journavx targets pain-specific sodium channels underscores its potential to revolutionize acute pain management, offering clinicians an effective tool that circumvents the pitfalls associated with opioid therapies.
Comparative Analysis and Additional Mechanistic Insights
In addressing the merits of Journavx, it is imperative to consider both its innovative mechanism and its clinical implications relative to traditional opioid analgesics.
- *Contrast with Opioid Mechanisms: *
Opioids, such as morphine and fentanyl, achieve analgesia primarily through the activation of μ-opioid receptors, leading to the inhibition of neurotransmitter release and modulation of pain perception within the central nervous system. Despite their efficacy, opioids are accompanied by significant risks, including respiratory depression, tolerance development, and potential for addiction. Journavx, by contrast, exerts its effect peripherally—at the level of the nociceptive neuron—thus avoiding the central side effects that often complicate opioid therapy. - *Clinical Implications of a Sodium Channel Blocker: *
By directly targeting the ion channels responsible for the initiation and propagation of pain signals, Journavx offers a targeted approach that may be especially beneficial in postoperative pain and other acute pain scenarios. The selective blockade minimizes the risk of generalized neuronal inhibition, which can lead to unwanted side effects such as motor dysfunction or cognitive impairment. Additionally, the reduced likelihood of tolerance development presents a significant advantage over long-term opioid use. - *Extended Diagram: A Comparative Flowchart of Pain Signal Inhibition *
For further clarity, consider the following comparative flowchart:
[Pain Stimulus]
│
┌───────────┴───────────┐
│ │
[Opioid Pathway] [Journavx Pathway]
│ │
Activation of μ-Opioid Selective Blockade of
Receptors Voltage-Gated Sodium Channels
│ │
Inhibition of Neurotransmitter Stabilization of Inactivated State
Release at CNS in Peripheral Neurons
│ │
Altered Pain Perception Reduced Action Potential Firing
│ │
Potential Side Effects: Minimal CNS Side Effects,
Respiratory Depression, No Addiction Risk
Tolerance, Dependence- *Pharmacodynamic Considerations: *
The selective mechanism of Journavx allows for a rapid onset of action with an excellent safety margin. This targeted approach is anticipated to not only provide immediate relief from acute pain but also reduce the overall burden of side effects that can compromise patient recovery and well-being. Moreover, the peripheral action of Journavx suggests a lower propensity for central nervous system complications, an aspect that is particularly important in vulnerable populations such as the elderly or those with comorbid conditions. In our view, the precision and selectivity of Journavx’s sodium channel blockade represent a significant advancement in analgesic pharmacotherapy. The mechanistic differences between this agent and traditional opioids offer a promising pathway to effective pain management without the attendant risks of dependency and central adverse effects.
Conclusion, Future Directions, and Clinical Implications

In conclusion, Journavx’s approval marks a noteworthy milestone in the evolution of pain management therapies. Its mechanism of action—rooted in the selective inhibition of sodium channels essential for nociceptive signaling—demonstrates a sophisticated approach to attenuating pain that diverges markedly from opioid-based strategies. This final page synthesizes the insights discussed and offers an opinion on the broader impact of this therapeutic innovation.
***1. * ** **Synthesis of Mechanistic Insights: **
We have observed that Journavx operates by binding to a specific region on voltage-gated sodium channels, thereby stabilizing them in an inactivated state. This action directly interrupts the initiation and propagation of action potentials in peripheral pain fibers. The molecular specificity of Journavx not only ensures effective pain relief but also minimizes the risk of off-target effects—a stark contrast to the non-selective nature of many traditional analgesics.
*2. Clinical Impact and Therapeutic Advantages: *
The implications for patient care are profound. By circumventing the central nervous system mechanisms that underlie opioid addiction and tolerance, Journavx offers clinicians a valuable alternative in the treatment of acute pain, especially in postoperative settings. Its targeted approach could lead to improved patient outcomes, reduced hospital stays, and a lower incidence of drug-related complications. Furthermore, its efficacy in managing pain without engaging the opioid receptors holds promise for addressing the ongoing public health challenge posed by opioid misuse.
*3. Future Directions for Research and Development: *
While Journavx’s current clinical application is focused on acute pain management, future research may explore its utility in chronic pain conditions, neuropathic pain syndromes, and even in multimodal pain management strategies. The potential for developing analogues with enhanced selectivity or tailored pharmacokinetic profiles represents an exciting avenue for future investigation. In addition, further studies involving high-resolution structural imaging and molecular dynamics simulations will undoubtedly enrich our understanding of the drug’s binding interactions and inform the design of next-generation sodium channel blockers.
**4. Final Diagrammatic Summary: **
To encapsulate the entirety of our discussion, consider the following final diagram that integrates the key components of Journavx’s mechanism:
┌────────────────────────────────────────────────┐
│ Pain Signal Initiation │
├────────────────────────────────────────────────┤
│ Noxious Stimulus → Membrane Depolarization │
├────────────────────────────────────────────────┤
│ Voltage-Gated Sodium Channels (Na_V1.7, etc.) │
│ [Open → Inactivation → Refractory] │
├────────────────────────────────────────────────┤
│ Intervention with Journavx │
│ (Selective binding → Prolonged inactivated state)│
├────────────────────────────────────────────────┤
│ Outcome: Reduced Action Potential Firing │
│ → Attenuation of Pain Signal Transmission │
└────────────────────────────────────────────────┘5. Final Thoughts and Opinion:
In our considered opinion, the advent of Journavx represents a paradigm shift in the field of analgesia. Its innovative approach, targeting the very genesis of pain at the level of ion channel function, not only provides a much-needed alternative to opioids but also paves the way for the development of a new class of targeted pain therapies. The elegance of this mechanism—rooted in a deep understanding of neuronal physiology—epitomizes the fruitful convergence of molecular pharmacology and clinical medicine. We are optimistic that continued research and clinical application of such agents will significantly enhance patient care and contribute to resolving the pervasive challenges associated with pain management in modern medicine.
In summary, the detailed mechanism of Journavx as elucidated in these five pages offers a thorough understanding of its function and potential clinical benefits. We trust that this comprehensive explanation, replete with diagrammatic representations and in-depth analysis, provides valuable insights into this exciting advancement in non-opioid pain management.
- [ ]