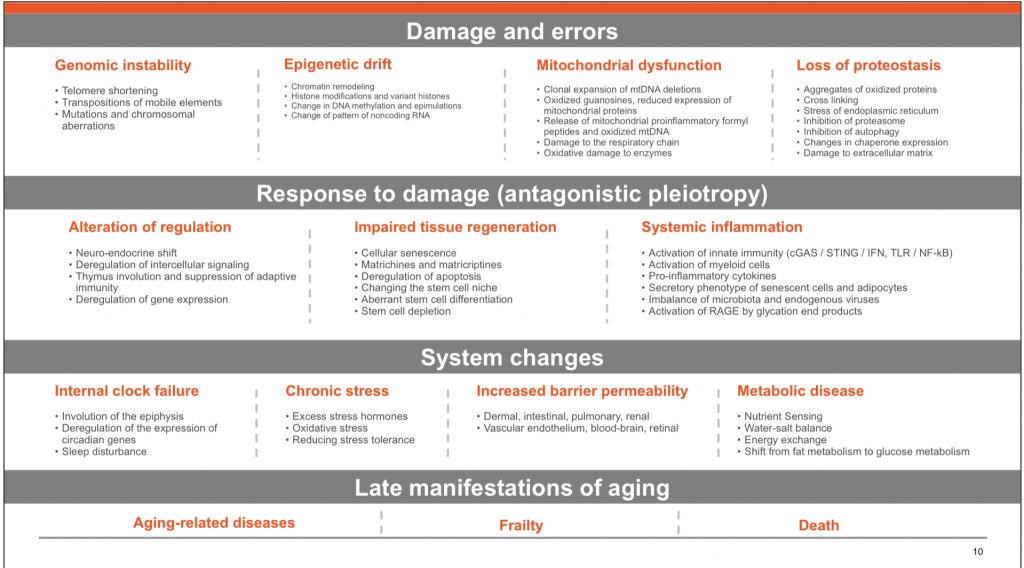
What is inflammation, and how is that induced and repairs or damage our tissues?
Inflammation is the body’s response to injury, infection, or irritation. It is a complex process involving various cells and molecules, including white blood cells, cytokines, and prostaglandins. Inflammation can be acute or chronic. Acute inflammation is a short-term response to injury or infection and is characterized by redness, swelling, warmth, and pain. Chronic inflammation, on the other hand, is a long-term response that can last for weeks, months, or even years and is associated with conditions such as arthritis, asthma, and autoimmune diseases.
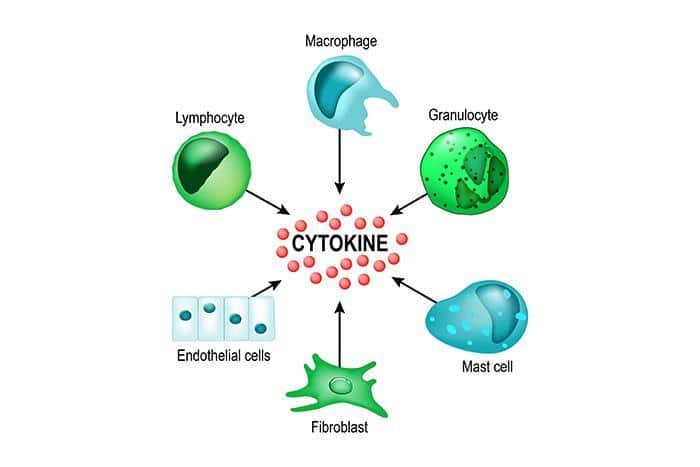
Inflammation is induced by various factors, including physical injury, infection, and exposure to toxins. When the body is injured or infected, immune cells are activated and release chemical signals called cytokines. These signals recruit more immune cells to the site of injury or infection, and these cells release other chemicals, such as prostaglandins, which cause the characteristic symptoms of inflammation.
Inflammation can repair or damage tissues depending on the cause and duration of the inflammation. Acute inflammation is a necessary and beneficial process that helps the body fight infection and injury by removing harmful agents and initiating the healing process. However, chronic inflammation can cause damage to healthy tissues and contribute to the development of chronic diseases such as cancer, heart disease, and Alzheimer’s disease.
![]()
What are acute and chronic inflammation, and how are they alike or different?
Acute inflammation and chronic inflammation are both types of inflammation, but they have some key differences.
Acute inflammation is a short-term response to injury or infection and is characterized by redness, swelling, warmth, and pain. It typically lasts for a few days to a few weeks. The immune system quickly sends white blood cells, such as neutrophils and macrophages, to the site of injury or infection. These cells remove harmful agents, such as bacteria or damaged tissue, and help to initiate the healing process. Acute inflammation is a necessary and beneficial process that helps the body fight infection and injury.
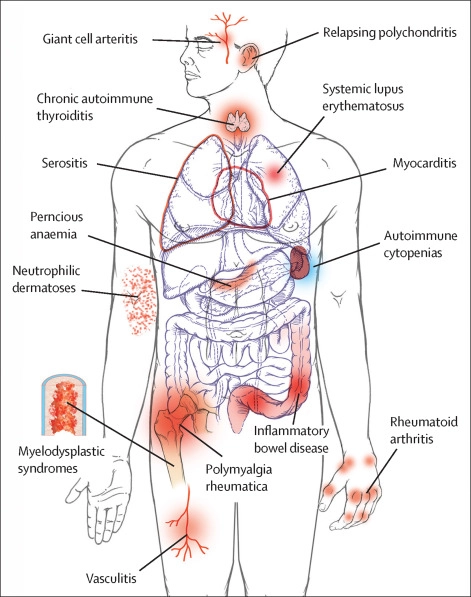
Chronic inflammation, on the other hand, is a long-term response that can last for weeks, months, or even years. It is associated with conditions such as arthritis, asthma, and autoimmune diseases. Chronic inflammation is characterized by a prolonged activation of the immune system, which can lead to damage to healthy tissues over time. Chronic inflammation can also increase the risk of developing chronic diseases such as cancer, heart disease, and Alzheimer’s disease.
Both acute and chronic inflammation involve the activation of the immune system and the release of chemical signals called cytokines, however, the duration and types of cells involved can be different. While acute inflammation tends to be more localized, chronic inflammation can be systemic, affecting multiple parts of the body.
In summary, acute inflammation is a short-term response that helps the body fight infection and injury, while chronic inflammation is a long-term response that can lead to damage to healthy tissues and increase the risk of chronic diseases.
![]()
How many types of immune cells are there in the body and how they work?
There are many different types of immune cells in the body, each with a unique function. The main types of immune cells are:
1- White blood cells (also called leukocytes) – These cells are the main cells of the immune system and are responsible for recognizing and attacking foreign invaders such as bacteria, viruses, and cancer cells. There are several types of white blood cells, including:
2- Neutrophils: These cells are the most abundant white blood cells and are the first to arrive at the site of infection or injury. They engulf and kill bacteria and other foreign particles.
3- Monocytes: These cells migrate to the site of injury or infection and mature into macrophages, which engulf and kill bacteria and other foreign particles.
4- Lymphocytes: These cells are responsible for recognizing and responding to specific pathogens. There are two main types of lymphocytes: B-lymphocytes and T-lymphocytes. B-lymphocytes produce antibodies, which are proteins that bind to and neutralize pathogens, while T-lymphocytes directly attack infected cells or cancer cells.
5- Macrophages: These cells are large white blood cells that can engulf and digest cellular debris, foreign particles and pathogens, and present antigens to other immune cells.
6- Dendritic cells: These cells are responsible for identifying and presenting antigens to T-lymphocytes, which then differentiate into cells that can directly attack infected cells or cancer cells.
7- Natural Killer cells: These cells are a type of T-lymphocyte that can directly attack and kill infected cells or cancer cells.
8- Mast cells: These cells are involved in allergic reactions and inflammation. They release histamine and other chemicals that cause the characteristic symptoms of an allergic reaction, such as redness, swelling, and itching.
All these cells work together in a coordinated manner to defend the body against foreign invaders and to maintain the overall health of the body. They are able to communicate with each other through chemical signals and physical interactions, allowing them to coordinate their efforts to effectively fight pathogens and promote healing.
![]()
Why immune cell go haywire and what are the consequences?
There are several reasons why immune cells can go haywire, including:
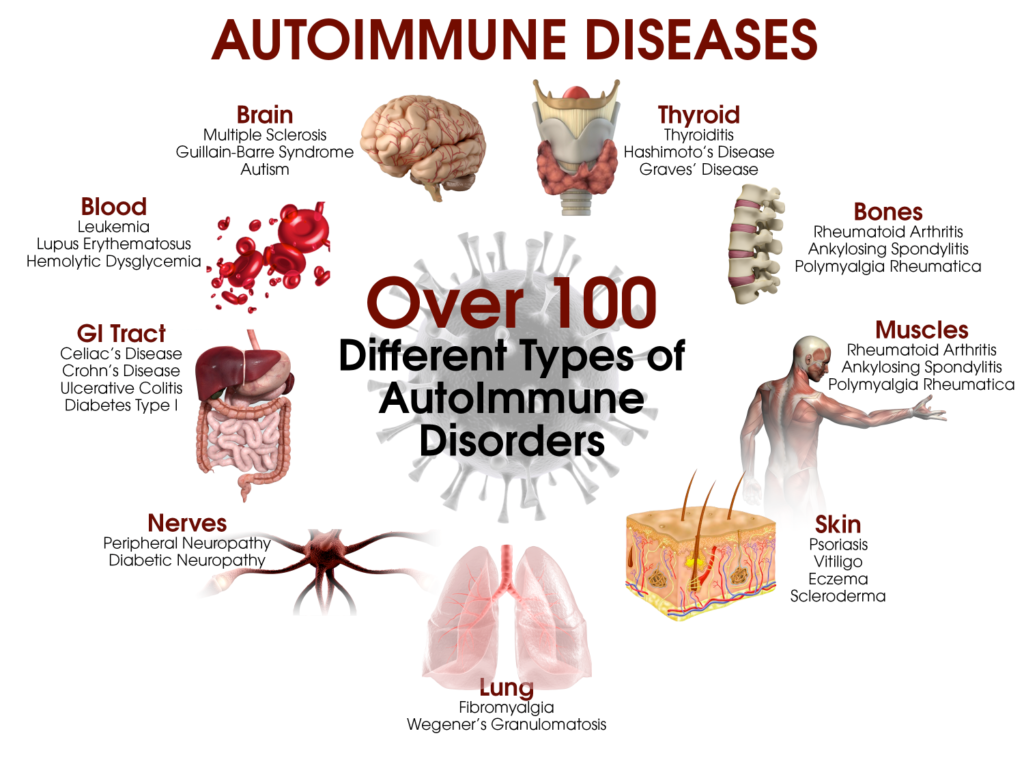
1- Genetic mutations: Certain genetic mutations can cause immune cells to become overactive or to malfunction, leading to conditions such as cancer or autoimmune diseases.
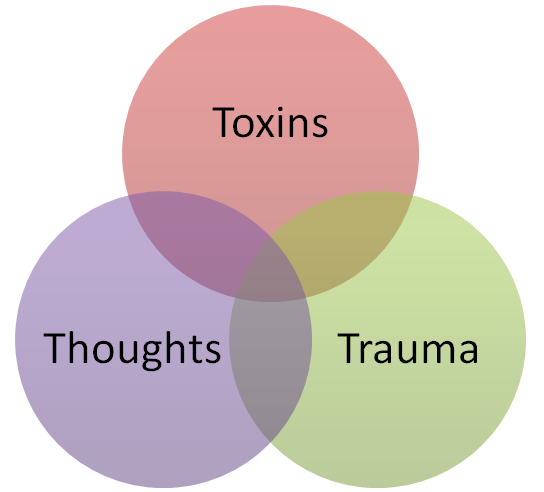
2- Environmental factors: Exposure to toxins, radiation, or certain viruses can damage immune cells and lead to dysfunction.
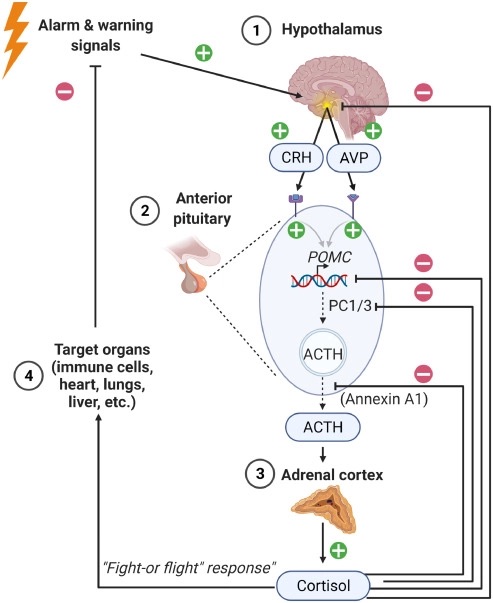
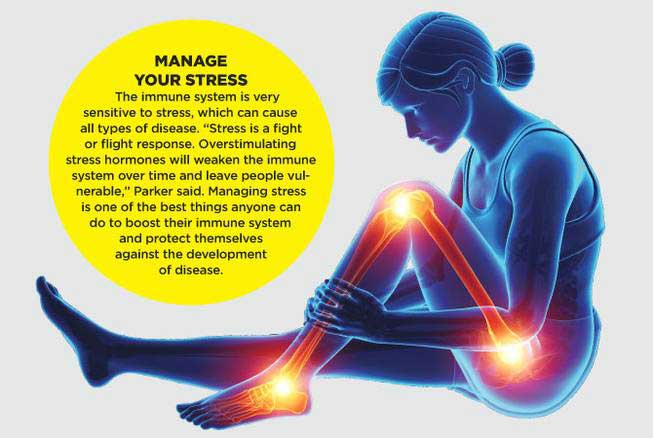
3- Chronic stress: Chronic stress has been linked to changes in the immune system, including the activation of inflammatory pathways, which can lead to dysfunction of the immune cells.
4- Aging: As we age, the immune system gradually loses its ability to function properly. This can increase the risk of infections, cancer, and autoimmune diseases.
When immune cells go haywire, they can cause a wide range of consequences, including:
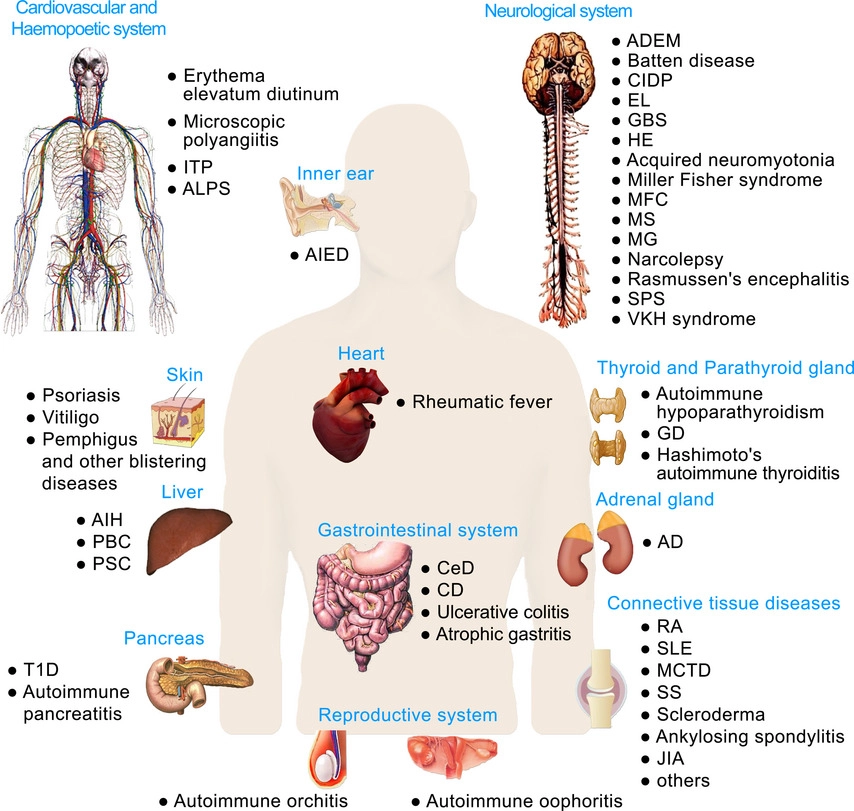
- Autoimmune diseases: When immune cells mistakenly attack the body’s own tissues, this can lead to conditions such as rheumatoid arthritis, lupus, and multiple sclerosis. 1- Cancer: When immune cells malfunction, they can allow cancer cells to grow and spread unchecked.
- 2- Chronic inflammation: Chronic inflammation can lead to damage to healthy tissues and increase the risk of chronic diseases such as cancer, heart disease, and Alzheimer’s disease.
- 3- Increased susceptibility to infection: When the immune system is not functioning properly, it can be more difficult to fight off infections.
- 4- Allergies: When the immune system overreacts to harmless substances, this can lead to allergic reactions such as hay fever, asthma, and eczema. Overall, a dysfunction of immune cells can lead to a wide range of health problems and it’s important to maintain a healthy lifestyle, including regular exercise, a healthy diet and stress management to help keep the immune system functioning properly.
![]()
Can you explain the healthy lifestyle, healthy diet and stress management?
Acute stress is a normal response to a sudden, unexpected event, such as a car accident or a natural disaster. It typically lasts for a short period of time and is resolved once the event is over.
Episodic stress refers to repeated or intermittent instances of acute stress. An example of episodic stress would be someone who frequently experiences acute stress due to work deadlines or financial problems.
Chronic stress is long-term stress that persists over an extended period of time. It can be caused by a variety of factors, such as a difficult job, a traumatic event, or a chronic illness. An example of chronic stress would be a person who has been dealing with a serious illness for an extended period of time.
A healthy lifestyle includes a variety of different practices and habits that can help promote overall health and well-being. This includes regular physical activity, maintaining a healthy diet, getting enough sleep, avoiding harmful substances such as tobacco and excessive alcohol, and managing stress.
Regular physical activity is important for maintaining a healthy immune system. Exercise can help improve circulation, which allows immune cells to move more quickly to sites of infection or injury. It can also increase the production of immune cells and improve their function.
Maintaining a healthy diet is also important for the immune system. Eating a diet that is high in fruits, vegetables, whole grains, and lean protein can provide the nutrients that are necessary for the immune system to function properly. Some specific nutrients that are important for the immune system include vitamin C, vitamin E, and zinc.
Getting enough sleep is important for the immune system because it is during sleep that the immune system is able to repair and regenerate. Chronic sleep deprivation can weaken the immune system and increase the risk of infections and other health problems.
Avoiding harmful substances such as tobacco and excessive alcohol can also help to keep the immune system functioning properly. Smoking and excessive alcohol consumption can damage immune cells and increase the risk of infections and other health problems.
Managing stress is also important for the immune system. Chronic stress can activate inflammatory pathways in the body, which can lead to dysfunction of immune cells. There are several ways to manage stress, including exercise, meditation, yoga, and therapy.
Overall, a healthy lifestyle can help to keep the immune system functioning properly and reduce the risk of infections and other health problems. It’s important to establish healthy habits and maintain them in the long run for the overall well-being of the body.
![]()
Innate and adaptive immune systems, how can be clarified?
The immune system is divided into two main branches: the innate immune system and the adaptive immune system.
The innate immune system is the body’s first line of defense against pathogens, such as bacteria and viruses. It is made up of a variety of cells and molecules that are always present in the body and are ready to respond quickly to invaders. The innate immune system is not specific to a particular pathogen, it does not “learn” from previous exposures to pathogens and does not have memory.
The innate immune system includes physical and chemical barriers, such as the skin and mucous membranes, as well as cells such as neutrophils, macrophages, and natural killer cells. These cells recognize and respond to pathogens using a variety of pattern recognition receptors, which are able to detect specific patterns that are found on the surface of pathogens. Once a pathogen is detected, the cells of the innate immune system release chemicals, called cytokines, which help to recruit other immune cells to the site of infection.
The adaptive immune system, on the other hand, is a more specific and targeted response to pathogens. It is made up of B-lymphocytes and T-lymphocytes. The adaptive immune system “learns” from previous exposures to pathogens, and has memory, which allows it to mount a stronger and more rapid response to a pathogen if it encounters it again. B-lymphocytes produce antibodies, which are proteins that bind to and neutralize pathogens, while T-lymphocytes directly attack infected cells or cancer cells.
Both innate and adaptive immune systems work together in a coordinated manner to defend the body against foreign invaders and to maintain the overall health of the body. The innate immune system acts as a first line of defense, quickly responding to invaders, while the adaptive immune system provides a more specific and targeted response that can eliminate pathogens more effectively.
![]()
New studies prove that some of the innate immune cells also learn? Recent studies have shown that certain cells of the innate immune system, such as macrophages and dendritic cells, can also exhibit a form of “memory” or “adaptive-like” behavior.
These cells have been found to be able to “remember” previous encounters with pathogens and to mount a stronger and more rapid response upon re-encountering the same pathogen. This is known as “trained immunity” or “innate immune memory”. The mechanism behind this process is not fully understood yet but it is thought to involve epigenetic changes that result in the modulation of gene expression in these cells.
This new findings suggest that the innate and adaptive immune systems are not as distinct as previously thought, but rather interact and overlap in their functions. This knowledge may help to inform new strategies for treating infectious diseases and cancer by targeting innate immune cells in addition to adaptive immune cells, which could result in more effective therapies.
It’s important to note that this is an active area of research and scientists are still trying to understand the exact mechanisms and implications of this phenomenon, further studies are needed to fully understand the implications of innate immune memory and how it can be harnessed for the development of new therapies.
![]()
Recent studies shows that innate immune cell live in tissues like heart, kidney, liver, and other tissues and from different functions along with defence
Recent studies have indeed shown that innate immune cells, such as macrophages and dendritic cells, can reside in various tissues in the body, including the heart, kidney, liver, and other organs. These cells are known as tissue-resident macrophages and dendritic cells, and they play a variety of important roles in maintaining the health of these tissues.
In the heart, for example, tissue-resident macrophages play a role in the repair and regeneration of cardiac tissue following injury. They have been shown to play a role in the formation of new blood vessels, the removal of cellular debris, and the modulation of inflammation.
In the liver, tissue-resident macrophages have been shown to play a role in the removal of cellular debris and the regulation of inflammation. They also play a critical role in the maintenance of the liver’s metabolic functions and the regulation of the immune response to pathogens.
In the kidneys, tissue-resident macrophages have been shown to play a role in the maintenance of renal function and the regulation of blood pressure. They also play a role in the removal of cellular debris and the regulation of inflammation.
In addition to defence, these cells have been found to play a key role in maintaining the health and function of various organs and tissues in the body. They are involved in a wide range of processes, such as tissue repair, regeneration, and the regulation of inflammation. These findings highlight the importance of tissue-resident macrophages and dendritic cells in maintaining overall health and suggest that targeting these cells could be a promising strategy for treating a wide range of diseases.
![]()
In heart they play a role in the heartbeat by electrical signling.
Yes, recent studies have shown that tissue-resident macrophages in the heart also play a role in the regulation of the heartbeat by modulating the electrical signaling of the heart.
Macrophages in the heart are known to produce a variety of signaling molecules that can influence the electrical activity of the heart. They can modulate the activity of ion channels, which control the flow of ions into and out of heart cells, and therefore modulate the electrical activity of the heart.
Some studies have also shown that macrophages in the heart play a role in the formation of new blood vessels and in the repair of damaged tissue following injury, which can help to maintain the health of the heart and improve its function.
Overall, macrophages in the heart, as well as other tissues, have been found to play a crucial role in maintaining the health of the heart and its function by modulating the electrical signaling and by repairing the damaged tissue. These findings highlight the importance of macrophages in the heart and suggest that targeting these cells could be a promising strategy for treating a wide range of heart diseases