Introduction
Cancer remains one of the most formidable adversaries in modern medicine, characterized by its relentless growth, ability to invade surrounding tissues, and capacity to metastasize to distant organs. Central to the persistence and progression of cancer is its adeptness at evading the body’s immune defenses. Traditionally, the mechanisms by which cancer cells outmaneuver immune cells have been attributed to genetic mutations, altered cell signaling, and the creation of an immunosuppressive tumor microenvironment. However, recent groundbreaking studies have unveiled a novel and insidious strategy employed by cancer cells: the hijacking of mitochondria from immune cells. This essay delves into the intricate biology of cancer cell metabolism, the role of mitochondrial DNA (mtDNA), the newly discovered mechanisms of mitochondrial transfer, expert insights, and the broader implications for cancer therapy and other diseases.
Understanding Mitochondria and mtDNA
Mitochondria, often referred to as the cell’s powerhouses, are essential organelles responsible for producing adenosine triphosphate (ATP) through oxidative phosphorylation, thereby supplying the energy necessary for various cellular functions. Unlike nuclear DNA, which resides within the cell nucleus and encodes the majority of genetic information, mitochondrial DNA (mtDNA) exists independently within the mitochondria in small, circular DNA molecules akin to bacterial genomes. This unique structure is a vestige of mitochondria’s evolutionary origins, supporting the endosymbiotic theory that posits mitochondria originated from free-living bacteria that entered into a symbiotic relationship with early eukaryotic cells.
One of the critical distinctions of mtDNA is its relative autonomy from nuclear DNA. While nuclear DNA contains sophisticated repair mechanisms to maintain genomic integrity, mtDNA lacks such robust systems, making it more susceptible to mutations. The mitochondrial environment, rich in reactive oxygen species (ROS) generated during ATP production, further exacerbates the vulnerability of mtDNA to genetic damage. Accumulated mutations in mtDNA can impair mitochondrial function, leading to deficiencies in energy production and contributing to various diseases, including cancer. In cancer cells, mtDNA mutations can drive metabolic reprogramming, facilitating rapid cell proliferation and survival under adverse conditions.
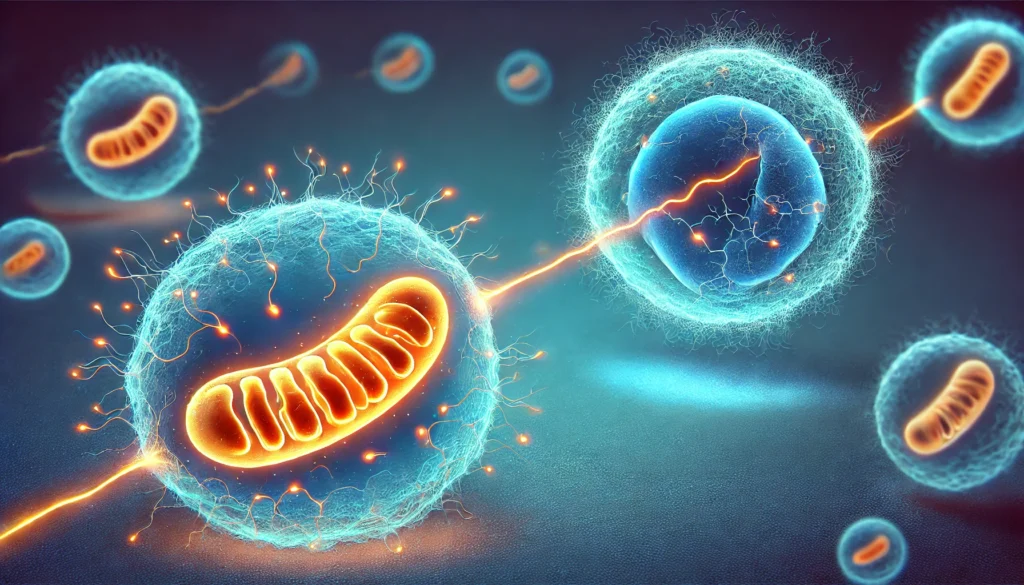
Mitochondrial Transfer: A Cellular Lifeline and a Double-Edged Sword
In recent decades, scientists have observed that mitochondria are not confined to their host cells but can be transferred between cells, a process known as mitochondrial shuttling. This phenomenon primarily serves as a cooperative mechanism where healthy cells donate mitochondria to struggling neighbors, aiding in tissue repair, immune responses, and cellular stress recovery. For instance, in tissue injury, stem cells may transfer mitochondria to damaged cells, enhancing their energy production and promoting regeneration.
However, early hints suggested that mitochondrial transfer could also play a role in cancer growth. Cancer cells, facing the high energy demands of rapid division and metastasis, often experience mitochondrial dysfunction due to mutations in their mtDNA. To compensate, these cells have developed strategies to acquire healthy mitochondria from their environment, thereby bolstering their metabolic capabilities. This dual nature of mitochondrial transfer—as both a supportive mechanism and a tool for malignancy—highlights its complex role in cellular biology and disease progression.
Cancer Cells’ Mitochondrial Hijacking: Mechanisms and Implications
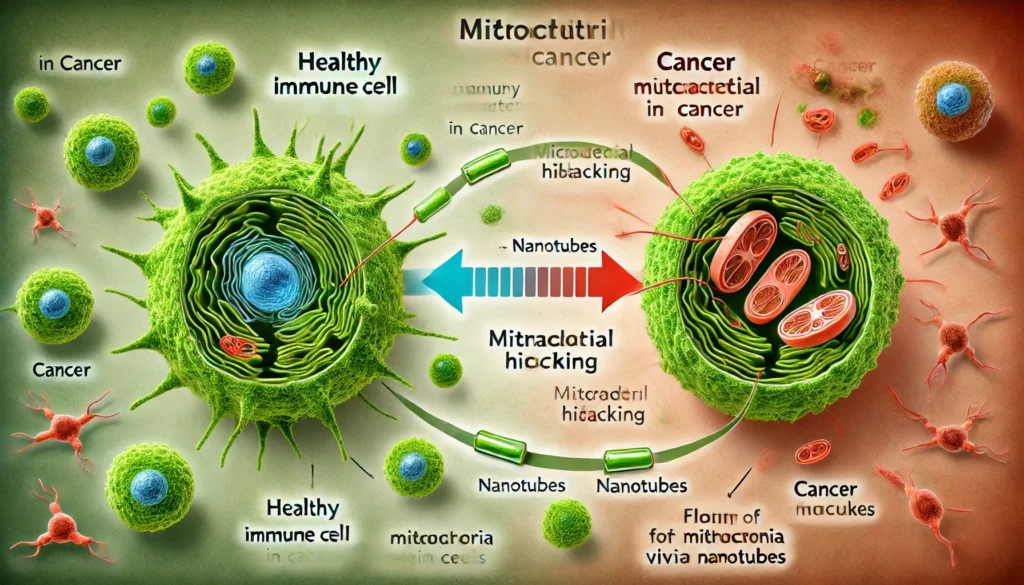
A landmark study published in Nature has shed light on the sophisticated methods cancer cells employ to hijack mitochondria from immune cells, thereby undermining the body’s defenses. The research revealed that cancer cells extend nanotubes—microscopic, tube-like structures—that penetrate immune cells, extracting healthy mitochondria. This theft not only depletes immune cells of their energy sources but also strengthens cancer cells by enhancing their mitochondrial function. Concurrently, cancer cells emit their own damaged mitochondria encapsulated in fatty vesicles toward immune cells. These defective mitochondria generate excessive ROS, inducing oxidative stress and apoptosis in immune cells, effectively weakening the immune response against the tumor.
As the research team observed, cancer-derived mtDNA can almost completely replace native mtDNA in immune cells, disrupting their normal metabolic functions and impairing their ability to generate ATP. This genetic takeover transforms immune cells, reducing their efficacy in targeting and eliminating cancer cells. Stanford’s Holden Maecker described the findings as “sounds crazy, like science fiction,” emphasizing the revolutionary nature of this discovery. Similarly, Jonathan Brestoff from Washington University School of Medicine highlighted that this mitochondrial swapping is a newly discovered mechanism that thwarts anticancer defenses, raising questions about its prevalence in other diseases.
Expert Insights and Broader Implications
The scientific community has responded with a mixture of astonishment and intrigue to these revelations. Experts like Maecker and Brestoff acknowledge that the discovery challenges existing paradigms of mitochondrial biology and cellular interaction. The realization that mitochondria can be mobile entities, capable of intercellular transfer, opens up new avenues for understanding not only cancer but also a range of other diseases where cellular communication and energy dynamics are disrupted.
In neurodegenerative diseases such as Parkinson’s and Alzheimer’s, impaired mitochondrial function is a hallmark feature. If neurons or glial cells engage in mitochondrial transfer, disruptions in this process could exacerbate neuronal loss and dysfunction. Similarly, in cardiovascular diseases, the transfer of mitochondria between cardiac cells and endothelial cells might influence tissue repair and regeneration following ischemic injury. Understanding whether and how mitochondrial shuttling operates in these contexts could reveal novel therapeutic targets aimed at restoring proper mitochondrial function and intercellular communication.

Therapeutic Implications: Targeting Mitochondrial Transfer
The discovery of mitochondrial hijacking by cancer cells offers profound insights into potential therapeutic interventions. By targeting the mechanisms of mitochondrial transfer, such as inhibiting nanotube formation or blocking the signals that facilitate mitochondrial uptake, researchers could effectively starve cancer cells of the additional energy resources they commandeer from immune cells. Additionally, therapies designed to prevent cancer cells from releasing damaged mitochondria could mitigate the oxidative stress imposed on immune cells, enhancing their ability to resist tumor-induced dysfunction.
One promising approach involves the use of mitochondrial stabilizers to protect immune cell function, thereby preserving their energy supply and boosting their capacity to combat cancer. Another strategy could involve the development of molecules that selectively target and neutralize cancer-derived mitochondria, preventing them from compromising immune cells. Furthermore, enhancing the natural ability of immune cells to retain and utilize their mitochondria could restore the balance between immune surveillance and tumor evasion.
Case Studies and Examples
To illustrate the practical implications of these findings, consider the case of metastatic breast cancer. Patients with metastatic breast cancer often exhibit resistance to conventional therapies, partly due to the tumor’s ability to manipulate the immune microenvironment. By applying therapies that disrupt mitochondrial transfer, such as inhibitors of nanotube formation, researchers could potentially restore immune cell functionality, making immunotherapies more effective in targeting and eliminating metastatic cells.
Another example lies in the realm of regenerative medicine. In conditions like myocardial infarction, where cardiac tissue is damaged, promoting the transfer of healthy mitochondria to cardiac cells could enhance tissue repair and improve patient outcomes. Conversely, in autoimmune diseases like rheumatoid arthritis, where immune cells are erroneously attacking healthy tissue, preventing the transfer of mitochondria could help mitigate the disease’s progression by preserving immune cell function.
Conclusion: The Future of Mitochondrial Research in Cancer and Beyond
The revelation that cancer cells can hijack mitochondria from immune cells marks a pivotal advancement in our understanding of tumor biology and immune evasion. This discovery not only challenges the traditional view of mitochondrial immobility but also highlights the intricate and multifaceted strategies cancer cells employ to sustain their growth and undermine the body’s natural defenses. As scientists continue to explore the prevalence and mechanisms of mitochondrial exchange, the potential applications of this knowledge extend beyond oncology, offering new insights into a range of diseases characterized by disrupted cellular communication and energy dynamics.
The path forward involves a concerted effort to unravel the molecular underpinnings of mitochondrial transfer, identify key regulatory pathways, and develop targeted therapies that can disrupt the harmful exchanges between cancer cells and immune cells. By leveraging advanced imaging technologies, genetic engineering, and molecular biology techniques, researchers are poised to unlock the full potential of mitochondrial dynamics in disease management and therapy.
In essence, the study of mitochondrial hijacking exemplifies the ever-evolving landscape of cancer research, where each discovery opens new doors and poses new questions. It underscores the importance of looking beyond conventional mechanisms to uncover the hidden layers of cellular interaction that drive disease progression. As our understanding deepens, so too does our ability to devise innovative and effective treatments, ultimately bringing us closer to overcoming the challenges posed by cancer and other complex diseases.

Takeaways and Future Directions
The exploration of mitochondrial transfer in cancer biology serves as a compelling reminder of the complexity of cellular interactions and the ingenuity of cancer cells in adapting to their environment. Key takeaways from this comprehensive examination include:
1. Mitochondrial Autonomy and Vulnerability: Understanding the unique characteristics of mtDNA, including its circular structure and susceptibility to mutations, is crucial in comprehending how mitochondrial dysfunction contributes to cancer progression.
2. Dual Nature of Mitochondrial Transfer: While mitochondrial shuttling can be a beneficial mechanism for cellular repair and regeneration, its exploitation by cancer cells highlights the delicate balance between cooperation and manipulation in the cellular ecosystem.
3. Innovative Therapeutic Targets: The identification of mitochondrial hijacking mechanisms opens up new therapeutic avenues, such as targeting nanotube formation, inhibiting mitochondrial transfer, and protecting immune cell mitochondria, which could enhance the efficacy of existing cancer treatments.
4. Broader Implications for Other Diseases: The principles of mitochondrial exchange have far-reaching implications beyond cancer, potentially impacting the understanding and treatment of neurodegenerative diseases, cardiovascular disorders, and autoimmune conditions.
5. Interdisciplinary Research: Addressing the complexities of mitochondrial dynamics requires a multidisciplinary approach, integrating insights from cellular biology, genetics, immunology, and bioengineering to develop comprehensive solutions.
Example: CAR T-Cell Therapy Enhancement
Chimeric Antigen Receptor (CAR) T-cell therapy has emerged as a revolutionary treatment for certain types of cancer. However, its effectiveness can be hampered by the immunosuppressive tumor microenvironment. By incorporating strategies to prevent mitochondrial hijacking, such as using inhibitors that block nanotube formation, CAR T-cells could maintain their energy supply and functionality within the tumor, enhancing their ability to target and destroy cancer cells more effectively.
Example: Mitochondrial Protection in Autoimmune Diseases
In autoimmune diseases like multiple sclerosis, where immune cells mistakenly attack healthy nerve cells, protecting the mitochondria of these immune cells from being compromised by external sources could help maintain their proper function and prevent unwarranted immune responses. This approach could lead to novel treatments that specifically safeguard mitochondrial integrity, reducing the severity of autoimmune attacks without broadly suppressing the immune system.
Final Thoughts
The intricate interplay between cancer cells and immune cells through mitochondrial transfer underscores the complexity of cancer biology and the sophisticated strategies employed by tumors to thrive. As research continues to uncover the depths of mitochondrial dynamics, the integration of these insights into clinical practice holds the promise of more effective and targeted therapies. Embracing this knowledge not only enhances our understanding of cancer but also equips us with the tools to tackle a myriad of other diseases, ultimately advancing the frontiers of medical science and improving patient outcomes.