Dear Reader,
The recent research findings regarding sulforaphane—a bioactive compound predominantly found in broccoli sprouts—merit considerable attention, particularly in the context of prediabetes and the broader trajectory toward type 2 diabetes. I shall provide an extensive examination of the topic, incorporating both the mechanistic insights and the broader implications for personalized treatment approaches.
Background on Sulforaphane
Sulforaphane is a naturally occurring isothiocyanate, celebrated for its potent antioxidant properties. Derived largely from cruciferous vegetables such as broccoli sprouts, this compound has been the subject of numerous studies due to its ability to induce phase II detoxification enzymes and modulate the body’s endogenous antioxidant defenses. Its role in activating the Nrf2 (nuclear factor erythroid 2–related factor 2) pathway is especially notable; this pathway orchestrates the expression of a battery of cytoprotective genes that mitigate oxidative stress—a key underlying factor in many metabolic disorders.
Prediabetes and Its Clinical Significance
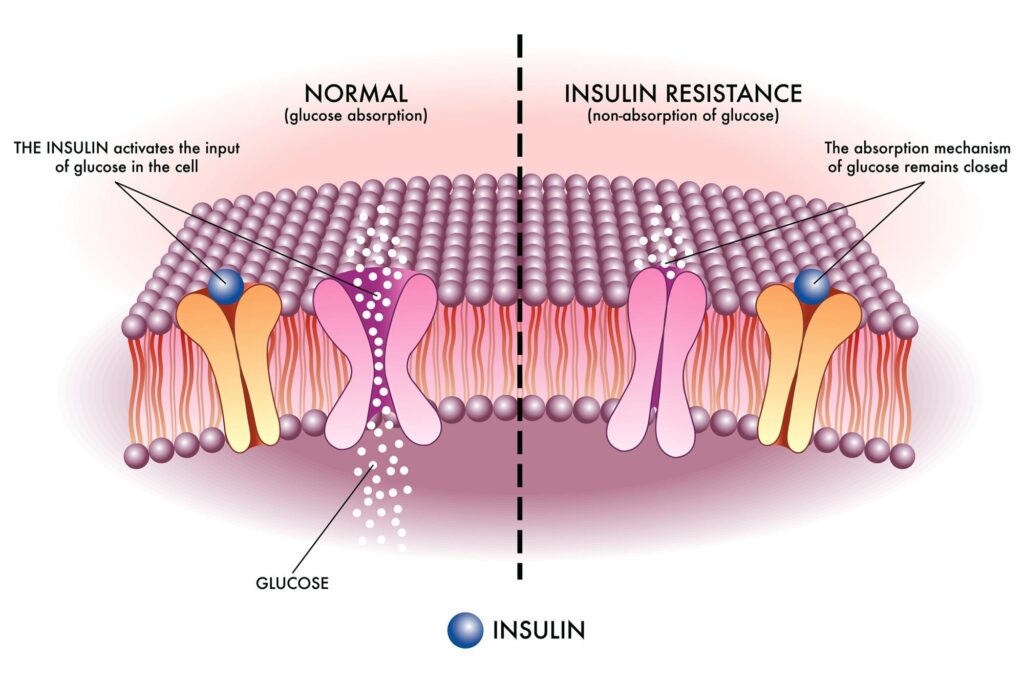
Prediabetes represents a metabolic state characterized by elevated blood glucose levels that, while not yet reaching the threshold of type 2 diabetes, indicate a heightened risk for its development. The condition is multifactorial, influenced by genetic predispositions, lifestyle factors, and environmental exposures. It is within this clinical window that intervention can be particularly impactful, potentially forestalling the progression to full-blown diabetes. However, the heterogeneous nature of prediabetes—wherein patients may exhibit varying degrees of insulin resistance, beta-cell dysfunction, and inflammatory markers—necessitates a tailored approach to treatment.
Insights from Recent Research
The study under discussion offers promising evidence that sulforaphane can improve blood sugar levels in a subset of individuals with prediabetes. This observation is significant for several reasons:
- Mechanistic Underpinnings:
Sulforaphane’s activation of the Nrf2 pathway not only enhances cellular antioxidant defenses but also appears to exert a modulatory effect on metabolic processes. By reducing oxidative stress, sulforaphane may help to preserve pancreatic beta-cell function and improve insulin sensitivity, thereby contributing to better glycemic control. - Personalized Medicine Implications:
The research underscores an emerging paradigm in metabolic health management: the need for personalization. Given that not all individuals with prediabetes respond uniformly to a given intervention, the efficacy of sulforaphane may depend on specific patient characteristics, such as genetic markers, baseline metabolic status, or even gut microbiota composition. Such findings advocate for a precision medicine approach, wherein interventions are tailored based on individual biological profiles rather than a one-size-fits-all model.

- Broader Therapeutic Potential:
Beyond glycemic control, sulforaphane’s antioxidant and anti-inflammatory effects suggest that it may have a wider therapeutic role. These properties are beneficial not only for mitigating the oxidative stress that contributes to the pathogenesis of diabetes but also for addressing other conditions where inflammation and oxidative damage play a critical role.
Critical Analysis and Considerations While the study’s findings are promising, several factors must be taken into account:
• Heterogeneity of Response:
It is essential to recognize that the observed benefits were limited to “some people” with prediabetes. This variability in response highlights the complexity of metabolic regulation and the influence of inter-individual differences. Consequently, further research is required to delineate which subpopulations are most likely to benefit from sulforaphane supplementation and to understand the underlying determinants of this responsiveness.
• Dose and Bioavailability:
Another pertinent consideration is the dose of sulforaphane required to elicit a measurable improvement in blood sugar levels. The bioavailability of sulforaphane can vary considerably depending on the form in which it is consumed (e.g., raw versus cooked broccoli sprouts) and individual differences in digestive and metabolic processing. Optimizing the delivery method and dosage will be critical in translating these findings into clinical practice.
• Integration into a Comprehensive Treatment Regimen:
While sulforaphane shows potential as a therapeutic adjunct, it is unlikely to serve as a stand-alone treatment for prediabetes. A comprehensive approach, including dietary modifications, physical activity, and possibly other pharmacological interventions, remains essential for effective metabolic management. The notion of “personalized treatment” should, therefore, be understood as a multifaceted strategy that integrates novel nutraceutical interventions with established lifestyle and medical therapies.
Personal Reflections and Future Directions In my considered opinion, the exploration of sulforaphane as a therapeutic agent in the context of prediabetes represents an exciting frontier in metabolic research. The shift toward personalized medicine—where treatment is tailored to the unique genetic, metabolic, and environmental contexts of each individual—is both scientifically justified and clinically promising. However, it is incumbent upon the scientific community to approach these findings with a balanced perspective. While the data are encouraging, they also remind us of the inherent complexity of human metabolism and the necessity for rigorous, large-scale studies before definitive clinical recommendations can be made. For clinicians and researchers alike, this study provides both a challenge and an opportunity: a challenge to unravel the nuanced mechanisms by which sulforaphane exerts its effects, and an opportunity to refine our approach to prediabetes treatment in a way that is both innovative and individualized.Wrap Up
In summary, the research suggesting that sulforaphane can improve blood sugar levels in some individuals with prediabetes is an important contribution to our evolving understanding of metabolic health. It reinforces the need for personalized treatment strategies that account for the heterogeneity of prediabetes and highlights the potential of natural compounds, such as sulforaphane, in augmenting traditional treatment modalities. As we look toward the future, continued research and clinical trials will be essential in validating these findings and in determining how best to integrate such interventions into a holistic and patient-centered framework. I trust that this detailed analysis has provided you with a comprehensive understanding of the subject, and I remain optimistic about the promising avenues for further exploration in personalized metabolic therapy.
